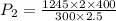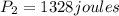As we know by ideal gas equation that
PV = nRT
here
n = number of moles
R = universal gas constant
T = temperature
P = pressure
V = volume
now here we have two set of data for same number of moles
so we will have
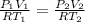
now we have

now we have to solve it for pressure