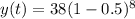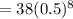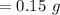Answer:
0.15 g of Polonium will be in the sample 1104 days later.
Explanation:
The exponential function for decay is,

Where,
y(t) = the amount after time t,
a = initial amount = 38 g
r = rate of decay = 50% = 0.50 (as the sample is getting halved each time)
t = time period =
 (as we have to convert the time in terms of half lifes)
(as we have to convert the time in terms of half lifes)
Putting all the values,