Answer:
D) all of the above
Step-by-step explanation:
As per ideal gas equation we know that

here we know
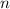 = number of moles
= number of moles
R = ideal gas equation
T = temperature
V = volume of the gas
so if we rearrange the equation then we know
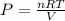
so pressure depends on all factors
Volume, Temperature and number of particles