Answer:
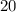 moles of CO2 can be produced from a reaction of 10.0 moles C2H6
moles of CO2 can be produced from a reaction of 10.0 moles C2H6
Step-by-step explanation:
In this reaction -
2 moles of C₂H6 produces four molecules of Carbon dioxide (CO2)
So 1 mole of C₂H6 will produce
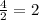 moles of Carbon dioxide (CO2)
moles of Carbon dioxide (CO2)
Thus, 10 moles of C₂H6 will produce
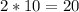 moles of Carbon dioxide (CO2)
moles of Carbon dioxide (CO2)