Answer:
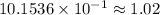 grams.
grams.
Explanation:
We have been given that a closed container has
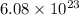 atoms of a gas. Each atom of the gas weighs
atoms of a gas. Each atom of the gas weighs
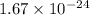 grams.
grams.
To find the total mass, in grams, of all the atoms of the gas in the container, we will multiply number of atoms with mass of each atom.
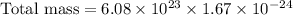
Using exponent property
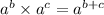 , we will get:
, we will get:
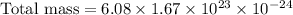
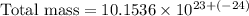
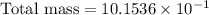
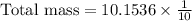

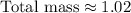
Therefore, the approximate total mass, in grams, of all the atoms of the gas in the container is
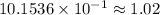 grams.
grams.