Answer : The number of moles of oxygen needed to burn are, 1.995 moles
Solution : Given,
Moles of methyl alcohol,
 = 1.33 mole
= 1.33 mole
The given balanced chemical reaction is,
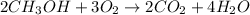
From the balanced reaction, we conclude that
As, 2 moles of methyl alcohol react with 3 moles of oxygen
So, 1.33 moles of methyl alcohol react with
 moles of oxygen
moles of oxygen
Therefore, the number of moles of oxygen needed to burn are, 1.995 moles