Answer:
113.6567 kiloJoules of heat is evolved or absorbed when 10.0 g of lithium iodide completely dissolves in water.
Step-by-step explanation:
Lithium iodide has a lattice energy =
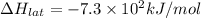
Lithium iodide has a heat of hydration =
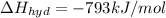
Heat of the solution of lithium iodide :
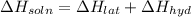
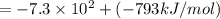
= -1,523 kJ/mol
Mass of lithium iodide = 10.0g
Moles of lithium iodide =
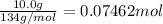
Heat of the solution of lithium iodide when 0.07462 mol is dissolved: Q
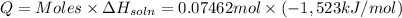
Q = -113.6567 kJ
(Negative sign indicates that energy released which means that temperature of the solution after dissolving lithium iodide will increase.)