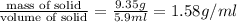Answer: 1.58 g/ml
Explanation:
Given :Total mass= 17.33 g
Mas of solid = 9.35 g
Thus Mass of benzene = Total mass - Mass of solid= (17.33-9.35)g= 7.98 g
Density of benzene = 0.876 g/ml
Therefore Volume of benzene =
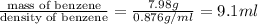
Total volume = 15 ml
Volume of solid = Total volume - Volume of benzene= (15-9.1)=5.9ml
Thus Density of solid=