Answer: The radiation has a frequency of
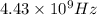 and is a type of radio wave.
and is a type of radio wave.
Step-by-step explanation:
The equation given by Planck's follows:

where,
E = energy of the light =
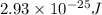
h = Planck's constant =
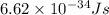
 = frequency of light = ?
= frequency of light = ?
Putting values in above equation, we get:
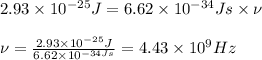
The relation between frequency and wavelength is given as:

where,
c = the speed of light =
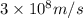
 = frequency of radiation =
= frequency of radiation =
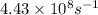
 = wavelength of the radiation = ?
= wavelength of the radiation = ?
Putting values in above equation, we get:
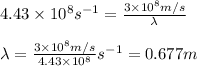
The radiation having wavelength 0.677 m belongs to radio waves.
Hence, the radiation has a frequency of
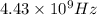 and is a type of radio wave.
and is a type of radio wave.