Answer:
pH=8.32
Step-by-step explanation:
The relevant equilibrium for this problem is
F⁻ + H₂O ↔ HF + OH⁻
With a constant Kb of
Kb=
![([HF][OH^(-)])/([F^(-)])](https://img.qammunity.org/2017/formulas/chemistry/high-school/1wtlfwvq6et5wb88z0fmt2vzrdvoizabuv.png)
Kb=
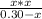
To calculate the value of Kb we use the formula Kw=Ka*Kb, where Kw is the ionization constant of water, 1 * 10⁻¹⁴.
1 * 10⁻¹⁴ = 7.2*10⁻⁴ * Kb
Kb = 1.4 * 10⁻¹¹
So now we have
1.4 * 10⁻¹¹=
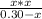
We make the assumption that x<<<0.30 M, so we can rewrite the equation of Kb as:
1.4 * 10⁻¹¹=
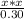
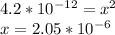
So [OH⁻]=2.05*10⁻⁶