Answer : The correct options are, (A), (C) and (D)
Explanation :
Le-Chatelier's principle : It states that if any change in the variables of the reaction, then the equilibrium will shift in that direction where the effect will be minimum.
The balanced equilibrium reaction will be,
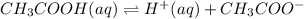
As the concentration of
 are decreased, the equilibrium will shift in a direction where concentration of
are decreased, the equilibrium will shift in a direction where concentration of
 is increasing and thus the dissociation of acid will increase to supply more of
is increasing and thus the dissociation of acid will increase to supply more of
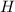 ions. During this process the reaction will move to the right direction.
ions. During this process the reaction will move to the right direction.
In the given equilibrium reaction when the
 are added then the
are added then the
 ion will combine with
ion will combine with
 ion to form water.
ion to form water.
Hence, the correct options are, (A), (C) and (D)