Answer: The correct answer is Option 1 and Option 3.
Step-by-step explanation:
We are given:
0.25 moles of

STP conditions states that 1 mole of a gas occupies 22.4 L of volume.
The volume occupied by all the gases at STP will be

According to mole concept: 1 mole of a compound contains
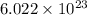 number of molecules or 1 mole of an element contains
number of molecules or 1 mole of an element contains
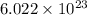 number of atoms.
number of atoms.
We know that the given gases are compounds. Thus, all the three gases will have
 number of molecules.
number of molecules.
Hence, the correct answer is Option 1 and Option 3.