Answer: The correct option is C.
Explanation: Ernest Rutherford proposed a gold-foil experiment.
In his experiment, he took a Gold foil & fired
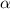 - particles (carrying +4 charge). He, then put a photogenic film around the gold foil to have a look where the particles are going. He thought that the alpha-particles will pass through the gold-foil because it was very thin.
- particles (carrying +4 charge). He, then put a photogenic film around the gold foil to have a look where the particles are going. He thought that the alpha-particles will pass through the gold-foil because it was very thin.
But to his surprise he observed that some particles bounced back. This observation will either mean that the alpha-particles are hitting something hard or hitting something that carries positive charge because positive charges repel each other.
Therefore, he made proposed that the nucleus is carrying some positive charge that consists of protons.
Hence, the correct option is C.