Answer : Yes, the concentrated
 is a catalyst in this reaction.
is a catalyst in this reaction.
Explanation :
Catalyst : It is a substance that increase the rate of chemical reaction but it is not consumed by the reaction. That means catalyst can be recovered chemically at the end of the reaction.
The given balanced chemical reaction is,
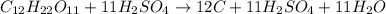
From the balanced chemical reaction we conclude that the amount of
 present in the reactant side are remains same as the amount of
present in the reactant side are remains same as the amount of
 present in the product side. That means the amount of
present in the product side. That means the amount of
 is not consumed by the reaction and it can be recovered chemically at the end of the reaction.
is not consumed by the reaction and it can be recovered chemically at the end of the reaction.
Hence, yes, concentrated
 is a catalyst in this reaction.
is a catalyst in this reaction.