Step-by-step explanation:
Since nitrogen is more electronegative than hydrogen therefore, it attracts the electron of hydrogen atom towards itself. Hence, the hydrogen atom acquires a negative charge in a molecule of ammonia
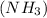 .
.
So, let the oxidation number of hydrogen in
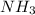 is x.
is x.
Therefore, calculate the oxidation number of hydrogen as follows.
Oxidation number of nitrogen +
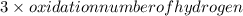 = charge on the molecule
= charge on the molecule
3 + 3x = 0
x =
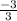
= -1
Thus, we can conclude that in the compound
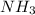 , nitrogen has an oxidation number of 3+ and hydrogen has an oxidation number of -1.
, nitrogen has an oxidation number of 3+ and hydrogen has an oxidation number of -1.