Answer: Option (A) is the correct answer.
Step-by-step explanation:
It is known that sodium chlorate is an ionic compound as the bond is formed between a metal and non-metal.
Therefore, when sodium chlorate is mixed in water then it will dissociate into ions. Also there is increase in temperature which will help in breaking ionic bond between the sodium and chlorate ions.
The chemical equation for the reaction will be as follows.
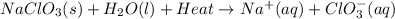
Therefore, we can conclude that solubility of sodium chlorate,
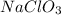 increases as the temperature of the water rises.
increases as the temperature of the water rises.