Answer: This leads to the formation of barium chromate and sodium nitrate.
Step-by-step explanation:
When barium nitrate reacts with sodium chromate, a yellow colored precipitate is formed which is known as barium chromate and also an aqueous solution of sodium nitrate is formed.
The chemical equation for the reaction of barium nitrate and sodium chromate follows:
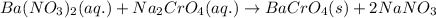
This reaction is a type of double displacement reaction because here, exchange of ions takes place.
Hence, this leads to the formation of barium chromate and sodium nitrate.