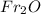Answer : The correct option is, (A)
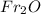
Explanation :
As we know that the francium is the alkali metal having the atomic number 87. The symbol of francium is, (Fr). The stable oxidation state of francium is (+1).
The element oxygen is the non-metal having the atomic number 8. The symbol of oxygen is, (O). The stable oxidation state of oxygen is, (-2).
When the francium combine with the oxygen, it forms francium oxide. It is an ionic compound in which the a metal react with the non-metal and combine with the an ionic bond by the criss-cross method.
Hence, the formula of the francium oxide is,