Answer: B) The volume will decrease.
Explanation: Boyle's Law: This law states that pressure is inversely proportional to the volume of the gas at constant temperature and number of moles.
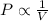 (At constant temperature and number of moles)
(At constant temperature and number of moles)

where,
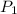 = initial pressure of gas = x
= initial pressure of gas = x
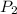 = final pressure of gas = say if doubles = 2x
= final pressure of gas = say if doubles = 2x
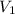 = initial volume of gas = v
= initial volume of gas = v
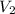 = final volume of gas =
= final volume of gas =
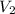
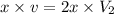

Thus volume will decrease.