Answer:
180.18 g/mol is the molar mass of glucose.
Step-by-step explanation:
Atomic weight of carbon = 12.01 g/mol
Atomic weight of oxygen = 16.0 g/mol
Atomic weight of hydrogen = 1.01 g/mol
Molar weight or mass of glucose =
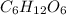
Molar mass of glucose :
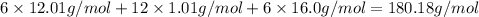
180.18 g/mol is the molar mass of glucose.