Answer: The chemical equation is written below.
Step-by-step explanation:
Sodium carbonate is an ionic compound and its dissociation will lead to the formation of its respective ions.
This compound is formed by the combination of sodium and carbonate ions.
The chemical equation for the dissociation of sodium carbonate follows:
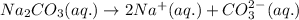
By Stoichiometry of the reaction:
1 mole of sodium carbonate produces 2 moles of sodium ions and 1 mole of carbonate ion.
Hence, the chemical equation is written above.