Answer:
The molar concentration of the unknown HCl is 2.5 M.
Step-by-step explanation:

Moles of NaOH in 25 mL of 2.0 M solution = n
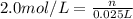
n = 0.05 moles
According to reaction 1 mol of NaOH reacts with 1 mol of HCl then 0.05 mol of NaOH will react with 0.05 moles of HCl.
Molar concentration of the unknown HCl solution.
[tex}M=\frac{\text{Number of moles of HCl}}{\text{Volume of solution in L}}[/tex]
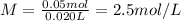
The molar concentration of the unknown HCl is 2.5 M.