Answer : The number of moles of nitrogen present in nitrous oxide are 3.22 moles.
Explanation : Given,
Mass of
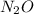 = 71.0 g
= 71.0 g
Molar mass of
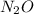 = 44 g/mole
= 44 g/mole
First we have to calculate the moles of
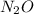 .
.
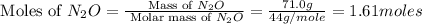
Now we have to calculate the moles of nitrogen in nitrous oxide.
In nitrous oxide
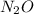 , there are 2 moles of nitrogen and 1 mole of oxygen.
, there are 2 moles of nitrogen and 1 mole of oxygen.
As, 1 moles of
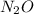 contains 2 moles of nitrogen
contains 2 moles of nitrogen
So, 1.61 moles of
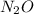 contains
contains
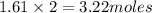 of nitrogen
of nitrogen
Therefore, the number of moles of nitrogen present in nitrous oxide are 3.22 moles.