Answer:
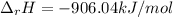
Step-by-step explanation:
Hello,
In this case of thermochemistry, the first step is to know all the involved species' enthalpies of formation as shown below (extracted from the NIST database):
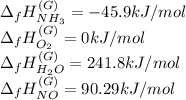
Now, for this particular chemical reaction and taking into account the involved stoichiometric coefficient, the feasible expression to compute the enthalpy change for that reaction is:
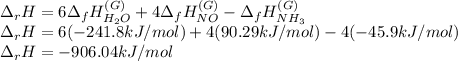
Best regards.