Answer:
potassium fluoride has highest boiling point
Step-by-step explanation:
- Potassium fluoride consists of
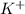 and
and
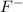 ions. Hence an ionic interaction is present in crystal of potassium fluoride as major intermolecular force.
ions. Hence an ionic interaction is present in crystal of potassium fluoride as major intermolecular force. - Glucose, water and methanol are polar protic covalent molecules. Hence hydrogen bonding force is present as a major force in those molecules.
- Ionic interaction force is much stronger than hydrogen bonding force.
- Therefore more heat energy is required to boil an ionic compound as compared to polar protic covalent molecules.
- Hence potassium fluoride has highest boiling point.