Answer: The correct option is A.
Explanation: Law of conservation of mass states that the total mass in a chemical reaction remains conserved that is total mass on the reactant side will always be equal to the product side.
We are given 4 chemical reactions, the total mass during a chemical reaction will be same in the case of reaction A because total number of atoms on the reactant side is equal to the total number of atoms on the product side.
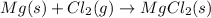
Mass of Magnesium = 24 g/mol
Mass of Chlorine = 35.5 g/mol
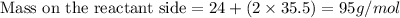
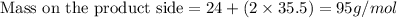
From the above, it is visible that the Total mass during this chemical reaction is same.
All the other reactions are not balanced, therefore the total mass will not be the conserved.