Answer:
0.5421 moles of carbon dioxide are emitted into the atmosphere.
Step-by-step explanation:
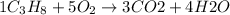
Moles of octane =
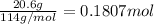
According to reaction 1 mol of octane gives 3 moles of carbon dioxide.
Then, 0.1807 moles of octane will give:
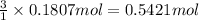 of carbon dioxide
of carbon dioxide
0.5421 moles of carbon dioxide are emitted into the atmosphere.