Answer:
the particles are moving faster
Step-by-step explanation:
As we know that diffusion means the rate of escape of gas from small opening.
So here the diffusion rate is basically related to the speed of gas molecules to escape out of the closed container.
So here rate of diffusion is given as
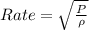
so it depends on pressure of gas and density of gas.
Also we know that gas molecules are loosely bounded to each other as we compare the bonding between molecules of liquid.
So here we will say that correct answer is
the particles are moving faster