Answer:
A whole number.
Step-by-step explanation:
Hello,
Empirical formula is the smallest representation of a chemical formula and the molecular formula is the actual formula of a given compound. For instance, glucose has the following molecular formula:
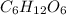
But its empirical formula is:
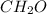
So they are relation by the 6 as a whole number.
Best regards.