Answer: 268.1 u
Step-by-step explanation:
Mass of isotope 1 = 267.8 u
% abundance of isotope 1 = 90.3% =
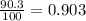
Mass of isotope 2 = 270.9 u
% abundance of isotope 2 = 9.7% =
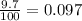
Formula used for average atomic mass of an element :
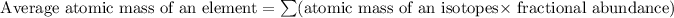
![A=\sum[267.8* 0.903+270.9* 0.097]](https://img.qammunity.org/2018/formulas/chemistry/high-school/neiunrbdxoek95bbkdxyu0dpvl06ot883w.png)

Therefore, the average atomic mass of an element is, 268.1 u