Answer : The correct option is, (C)
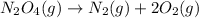
Explanation :
Decomposition reaction : It is a type of reaction in which a single compound decomposes into two or more new compounds.
The decomposition of dinitrogen tetraoxide into nitrogen gas and oxygen gas is shown by balanced chemical equation.
The balanced decomposition reaction will be,
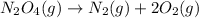
By the stoichiometry, 1 mole of dinitrogen tetraoxide decomposes into 1 mole of nitrogen gas and 2 moles of oxygen gas.
Hence, the correct option is, (C)