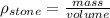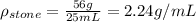Answer:
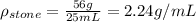
Step-by-step explanation:
Mass of the cylinder = 80 g
water added in the cylinder = 20 mL
mass of water + cylinder = 100 g
mass of water added = 100 g - 80 g = 20 g
density of water =
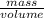
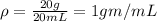
now when stone is put into the cylinder then the volume reached to 45 mL
so volume displaced by the stone = 45 mL - 20 mL
V = 25 mL
mass of stone = 156 g - 100 g = 56 g
now density of stone is