Answer : The mass of oxygen contained is, 0.1557 gram
Solution : Given,
Mass of potassium = 38.67 g
Mass of nitrogen = 13.86 g
First we have to calculate the mass of oxygen contained.
Mass of oxygen contained = 100 - (Mass of potassium + Mass of nitrogen)
Mass of oxygen contained = 100 - (38.67 + 13.86) = 47.47 g
Now we have to calculate the mass of oxygen contained in 328.0 mg or 0.328 g.
(1 g = 1000 mg)
In 100 gram of water, the mass of oxygen contained = 47.47 g
In 0.328 gram of water, the mass of oxygen contained =
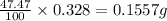
Therefore, the mass of oxygen contained is, 0.1557 gram