Step-by-step explanation:
It is known that on moving down a group there will occur a decrease in electronegativity of non-metals. Whereas stability of their conjugate bases increases on moving down the group.
This also means that their acid strength also increases.
For example,
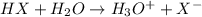
where, X = F, Cl, Br or I)
Therefore, acidity will increase in the following order.
HI > HBr > HCl > HF
Hence, the stability of their conjugate bases will be as follows.
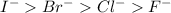
Thus, we can conclude that the most stable base is
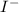 and least stable base is
and least stable base is
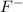 .
.