Answer: Option (D) is the correct answer.
Step-by-step explanation:
A chemical change is defined as the change which occurs due to change in chemical composition of a substance. A chemical change always leads to the formation of a new compound or substance.
For example, when an electric current passes through liquid water, hydrogen gas passes accumulates at one electrode and oxygen accumulates at the other.
Since, atoms of water are separating out. As a result, chemical properties will change. This means that a chemical change has occurred.
Also, bonds of water, (
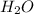 ) are breaking down. Hence, energy will be absorbed to break the bonds.
) are breaking down. Hence, energy will be absorbed to break the bonds.
Whereas energy is always released upon formation of bonds.
Thus, we can conclude that the statement a chemical change has occurred, with energy being absorbed, is true.