Answer : The mass number for this element is 125.
Explanation :
Isotope : It is defined as the element that have the same number of protons but have the different number of neutrons of each of the atom.
Atomic number is defined as the number of protons or number of electrons.
Atomic number = number of protons = number of electrons
Mass number is defined as the sum of number of protons and number of neutrons.
Number of neutrons = Mass number - Number of protons
As we are given :
Number of electrons in
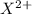 ion = 48
ion = 48
Number of neutrons in
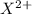 ion = 75
ion = 75
So, number of protons in
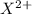 ion = 48
ion = 48
For neutral atom, the number of electrons will be:
Number of electrons = Number of protons in
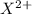 ion + Charge
ion + Charge
Number of electrons = 48 + (+2) = 48 + 2 = 50
Now we have to determine the mass number for this element.
Number of neutrons = Mass number - Number of protons
Mass number = Number of neutrons + Number protons
Mass number = 75 + 50
Mass number = 125
Therefore, the mass number for this element is 125.