Answer: Option (c) is the correct answer.
Step-by-step explanation:
It is known that kinetic energy is directly proportional to the temperature. Hence, mathematical relation between kinetic energy and temperature is as follows.
K.E =
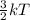
As kinetic energy defined as the energy occurring due to the motion of molecules of a substance.
Hence, it means that when there is increase in temperature then there will be increase in kinetic energy of molecules of gas. As a result, there will be more number of collisions between the atoms.
Hence, we can conclude that given molecules of gas and temperature are related as temperature increases, the atoms and molecules move farther apart.