Answer: The correct answer is Option A.
Step-by-step explanation:
To calculate the number of moles, we use the formula given by the equation:
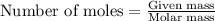
Where,
Given mass of
 = 454 grams
= 454 grams
Molar mass of
![CO_2=[12+(2* 16)]=44g/mol](https://img.qammunity.org/2018/formulas/chemistry/high-school/s5tj0lzhwii4hhsr4jjr2adnwt22xz581w.png)
Putting values in above equation, we get:
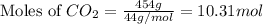
Hence, the correct answer is Option A.