Answer:
The reaction will proceed to the right (favoring the products).
Step-by-step explanation:
Let's consider the following isomerization reaction.
cis-2-butene ⇌ trans-2-butene
To predict in what direction will shift to reach equilibrium, we have to calculate the reaction quotient (Qp).
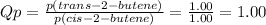
Since Qp (1.00) < Kp (3.40), the reaction will proceed to the right, so that the pressure of the product increases, the pressure of the reactant decreases, and Qp reaches the value of Kp.