Step-by-step explanation:
When we increase temperature of a substance then bond between the atoms will start to break and the molecules will move apart from each other. As a result, there will be increase in number of collisions between the molecules. Thus, reaction will occur at a faster rate.
Also, K.E =
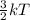
where k = boltzmann constant
T = temperature
Hence, kinetic energy is proportional to temperature. So, more is the temperature more will be the kinetic energy. Therefore, more will be the rate of reaction.