The decay function is
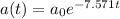
where
a₀ = initial mass
t = time, days
At half life, a(t) = a₀/2, therefore the time required to achieve half life is given by the equation
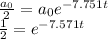
Take natural log of each side.
ln(1/2) = -7.571 t
Divide each side by -7.571 to obtain
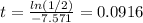
Answer: 0.0192 days (nearest thousandth)