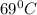Answer:
Step-by-step explanation:
Kinetic energy is the energy possessed by an object by virtue of its motion.
Average kinetic energy is defined as the average of the kinetic energies of all the particles present in a system. It is determined by the equation:
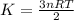
K= kinetic energy
n= number of moles
R= gas constant
T= temperature
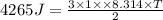
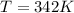
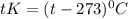
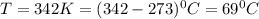
Thus temperature will be