Answer:
Step-by-step explanation:
In reaction:
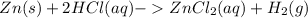
We know according to Le Chatelier's principle or "The Equilibrium Law" states that when a system experiences a disturbance (such as concentration, temperature, or pressure changes), it will respond to restore a new equilibrium state.
In this case rate of production of
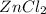 decreases when:
decreases when:
1. When concentration of HCl decreases.
2. When concentration of either
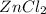 or
or
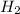 increases.
increases.
3. Increase in temperature because it is exothermic reaction.