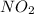Answer: The pair which shares the same empirical formula is
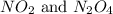 .
.
Step-by-step explanation: Empirical formula is defined as the chemical formula in which the elements in the compound are present in simplest ratio.
So, from the given pairs, only
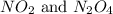 shares the same empirical formula which is
shares the same empirical formula which is