Answer: N is oxidized and O is reduced.
Step-by-step explanation:
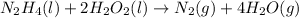
Oxidation: Removal of hydrogen or addition of oxygen is termed as oxidation.
Reduction: Removal of oxygen or addition of hydrogen is termed as reduction.
In a given reaction,
- Removal of hydrogen from
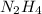 gives
gives
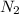 gas which means nitrogen is getting oxidized.
gas which means nitrogen is getting oxidized.
- Whereas removal of oxygen from
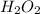 gives
gives
 gas which means that oxygen is getting reduced.
gas which means that oxygen is getting reduced.