Answer : The correct option is, (A) 52700 J
Solution :
Formula used :

Q = heat gained
m = mass of the substance = 198.5 g
c = heat capacity of water = 4.18 J/g°C
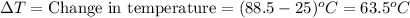
Now put all the given values in the above formula, we get
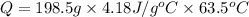
Q = 52687.855 J ≈ 52700 J
Therefore, the amount of heat required is, 52700 J