Answer: The balanced chemical equation is written below.
Step-by-step explanation:
A balanced chemical equation is defined as the equation in which total number of individual atoms on the reactant side is equal to the total number of individual atoms on the product side. These equations follow law of conservation of mass.
When hydrofluoric acid reacts with glass or silicon dioxide, it leads to the production of silicon tetrafluoride and water.
The chemical equation for the reaction of two follows:
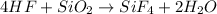
By Stoichiometry of the reaction:
4 moles of hydrofluoric acid reacts with 1 mole of silicon dioxide to produce 1 mole of silicon tetrafluoride and 2 mole of water.
Hence, the balanced chemical equation is written above.