Answer : Fluorine has Seven valence electrons.
Explanation :
Element = Fluorine
Atomic number = 9
Number of electrons = 9
Electronic configuration =
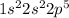
Electron structure is also known as electronic configuration. It is defined as the arrangement of electrons of an atom in an atomic orbitals.
According to Electron configuration, the 2s and 2p are the outermost energy shell and the number of electrons in 2s and 2p are 2 & 5 respectively. That means Fluorine has '7' valence electrons.
The electron structure of fluorine is shown below.