Answer: The correct answer is Carbon (C) and Lead (Pb)
Step-by-step explanation:
Valence electrons is defined as the electrons which are present in the outermost shell of the element.
For the given options:
Option 1: Oxygen (O) and Bromine (Br)
Electronic configuration of Oxygen :
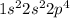
Electronic configuration of Bromine :
![[Ar]4s^23d^(10)4p^5](https://img.qammunity.org/2018/formulas/chemistry/high-school/24s4xb9tntdleug0cq4b4yah6kbwazb4b9.png)
Oxygen has 6 valence electrons and bromine has 7 valence electrons.
Option 2: Selenium (Se) and Bromine (Br)
Electronic configuration of Selenium :
![[Ar]4s^23d^(10)4p^4](https://img.qammunity.org/2018/formulas/chemistry/high-school/4w53q2nqm1r5gl7rap6m4xe59vea8ra8ra.png)
Electronic configuration of Bromine :
![[Ar]4s^23d^(10)4p^5](https://img.qammunity.org/2018/formulas/chemistry/high-school/24s4xb9tntdleug0cq4b4yah6kbwazb4b9.png)
Selenium has 6 valence electrons and bromine has 7 valence electrons.
Option 3: Carbon (C) and Lead (Pb)
Electronic configuration of Carbon :
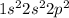
Electronic configuration of Lead :
![[Xe]6s^25d^(10)6p^2](https://img.qammunity.org/2018/formulas/chemistry/high-school/aw27at73xu6y26tlo61eoyyxot0plve8q3.png)
Carbon has 4 valence electrons and lead also has 4 valence electrons.
Option 4: Oxygen (O) and carbon (C)
Electronic configuration of Oxygen :
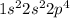
Electronic configuration of Carbon :
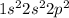
Oxygen has 6 valence electrons and carbon has 4 valence electrons.
Option 5: Sodium (Na) and Magnesium (Mg)
Electronic configuration of Sodium :
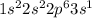
Electronic configuration of Carbon :
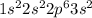
Sodium has 1 valence electrons and magnesium has 2 valence electrons.
From the above information, the correct answer is Carbon (C) and Lead (Pb)