The reaction above
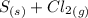
→
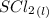
is a reduction-oxidation reaction in which one of the species was reduce (a decrease in oxidation state/ number) and the other oxidized (an increase in oxidation number/ state). This can be proven by finding the oxidation number of each species on the reactant and product side and compare them in order to decide which species was reduced and which was oxidized thus proving that the reaction really is a redox reaction.
Oxidation Number of Reactant:
S - ZERO [elements in uncombined form have oxidation number of zero]
Cl₂ - ZERO [diatoms have oxidation numbers of zero]
Oxidation Number of Product:
Cl in SCl₂ has an oxidation number of -1 [oxidation number of halogens are -1]
Since the chlorine oxidation number is found then we can write an
equation to solve for the oxidation number of sulfur
let Oxi. Number of S =

⇒

+ 2(-1) = 0 ; since compounds have oxi. number of zero
⇒

+ 2(-1) = 0
⇒

- 2 = 0
⇒

= + 2
Since the oxidation number of S moves from 0 to +2 (increase) then it implies that the species has been oxidized.
Since the oxidation number of Cl moves from 0 to -1 (decrease) then it implies that the species has been reduced.
Thus a REDOX reaction